Advances in cervical cancer vaccine- emerging prophylactic and therapeutic strategies
September 02, 2025 | Tuesday | Views
There is a critical need for new vaccine formulations with broader spectrum of efficacy and lower costs
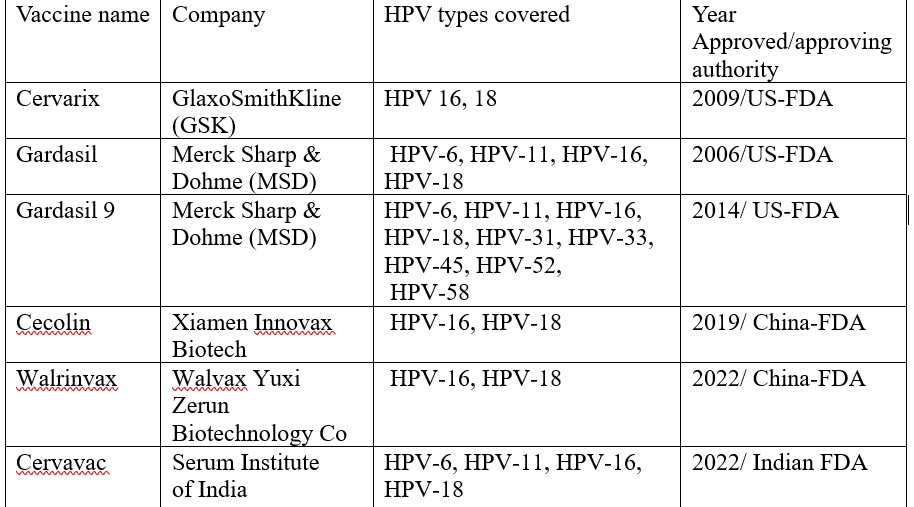
Cervical cancer is the fourth most common cancer worldwide. Despite the availability of prophylactic HPV vaccines, recent data indicates that the incidence and mortality of cervical cancer is increasing worldwide. As seen in the figure above, there are a few prophylactic vaccines approved for use. (All vaccines are administered by intramuscular injection)
Though these vaccines are highly effective, they provide type restricted immunity and hence incomplete protection. Moreover, there is risk of emergence of viral genotypes not included in the current vaccine formulations. Due to the complex and lengthy manufacturing process, the cost is high and hence not affordable by low-income populations. Hence, there is a critical need for new vaccine formulations with broader spectrum of efficacy and lower costs.
The following vaccine developments have taken place in the area of cervical cancer vaccines-
Prophylactic vaccines
L1 is the major capsid protein in HPV and represents 80% of the viral capsid proteins. Currently, the US FDA has licenced three L1 based vaccines - Cervarix, Gardasil, and Gardasil 9, that are produced using recombinant DNA technology in various hosts.
As the current VLP (virus-like particles) based L1 vaccines are expensive, to reduce the manufacturing cost, simpler expression systems such as E. coli have been employed in various trials.
Among the most recent and promising advancements in prophylactic HPV vaccines is the eleven-valent vaccine, (HPV-6/11/16/18/31/33/45/52/58/59/68) developed by the National Vaccine and Serum Institute in China. This vaccine is currently undergoing a Phase 3 clinical trial.
L2 , the minor capsid protein of HPV, is highly conserved across various HPV types, compared to L1 which is type-specific. Hence L2 based vaccines offer cross protection against multiple HPV genotypes. However, the L2 vaccines have weaker immune responses compared to L1 based vaccines. This is due to the linear structure of L2 and weaker T-helper cell activation.
Various preclinical platforms explored for L2 based vaccines include:
- L2 peptides delivered via bacteriophage VLPs (e.g. MS2, PP7): Bacteriophages can serve as efficient platforms for presenting L2 epitopes to enhance the immune response.
- L2 infused to hepatitis B core protein adenoviruses
- DNA and mRNA-based delivery of L2 sequences
- Recombinant bacteria can also be used to present the L2 protein
Only one L2 based vaccine has progressed to clinical trials
Therapeutic cervical cancer vaccine
These vaccines aim to stimulate the immune system to eliminate HPV infected cells or pre-cancerous cells. The following key developments have occurred in the area of therapeutic cervical cancer vaccine:
- AccumTM technology: This newly developed technology triggers a robust immune response as it allows the antigen to be delivered directly to the antigen presenting cells. This provides enhanced and long lasting protection. Trials done with Accum-E7 (aE7) based protein vaccine in mice by Bikorimana et al found that aE7 vaccination was superior to E7 vaccination alone. This technology has also been tested as a therapeutic vaccination in combination with immune checkpoint inhibitors with encouraging results
- DNA vaccines and adenovirus vectors: Han et al designed an adenoviral vector encoding a mutant HPV 16 e7 (Ad E7). The recombinant adenovirus is an ideal vector for delivering a therapeutic vaccine against cervical cancer. Trials have shown that DNA based adenoviral vector vaccines can effectively inhibit tumour growth and lead to tumour regression when used in conjunction with other therapeutic modalities.
- Lacticaseibacillus oral vaccine: Most vaccines for cervical cancer are administered by intramuscular route. Kawana et al investigated the potential of a therapeutic HPV 16 E7 expressing lacticaseibacillus based oral vaccine protein designed to be administered orally. They found that the vaccine had anti-tumour effects in patients with HPV 16 positive high grade cervical intraepithelial neoplasia. Oral route of vaccine administration offers an excellent alternative in the era of vaccine hesitancy and has the potential to improve compliance.
- mRNA vaccines- Research is ongoing on how mRNA vaccines can be used as a therapeutic treatment for cancer. mRNA vaccine codes for an antigen on the cervical cancer cells. It is encapsulated in lipid nanoparticles to facilitate its delivery to antigen presenting cells. Inside the cell, it travels to the ribosome and is translated into the target protein which is recognized by the antigen presenting cells.
The above mentioned strategies hold promise not only for providing broader protection against HPV genotypes but also improve the global vaccine coverage and compliance. Further trials are needed to study the full potential of these novel vaccine approaches.
Navya Mrig, Freelancer