How Immune Cell and Gene Therapies are Transforming Treatment in India
July 01, 2025 | Tuesday | Views | By Sudeep Krishna, Co-Founder & President, Healthark Insights
Immune cell and gene therapies are redefining treatment for cancer, genetic disorders, and autoimmune diseases in India. Once considered the realm of science fiction, these living medicines are now at the forefront of clinical care, offering hope for previously untreatable or poorly managed conditions.
Immuno-gene therapies represent a transformative frontier, combining genetic engineering with the body’s natural defenses to treat complex diseases like cancer and genetic disorders. Immuno-cell therapy boosts immune response by collecting, activating, and expanding a patient’s own immune cells outside the body, then reinfusing them to better target abnormal cells.
CAR-T cell therapy, one of the most advanced forms, modifies T cells with Chimeric Antigen Receptors (CAR) to identify and destroy cancer cells. In India, this segment is projected to grow from $710.9 million in 2024 to $2.5 billion by 2033, at a 15.1 per cent CAGR. CAR-NK therapy, another form, uses modified Natural Killer (NK) cells - either autologous or donor-derived - to target tumors, potentially offering safer and more effective outcomes.
In parallel, Gene Therapy modifies or replaces defective genes to treat cancer, inherited disorders, and chronic conditions. It includes Somatic gene therapy, which targets non-reproductive cells and affects only the treated person, and can be delivered ex-vivo (outside the body, then reinfused) or in-vivo (directly inside the body). Germline gene therapy alters reproductive cells or embryos, resulting in heritable genetic changes.
Immune cell and gene therapies increasingly intersect, with engineered immune cells like in CAR-T therapy offering better disease targeting and response durability. This convergence is advancing personalised medicine and unlocking new options for conditions once deemed untreatable.
Competitive Landscape in India
India’s cell and gene therapy ecosystem is expanding, with both established players and emerging innovators:
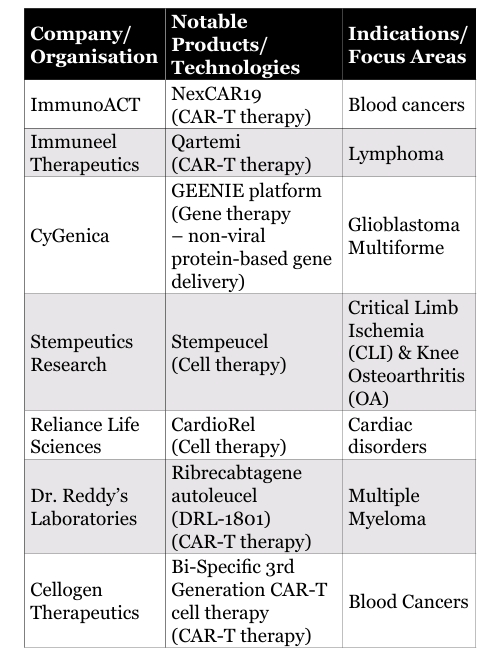
India’s first homegrown CAR-T therapy, developed by IIT Bombay and Tata Memorial Hospital, was approved in October 2023, marking a major milestone.
Strategic Partnerships and Collaborations
Recent collaborations are accelerating India’s cell and gene therapy landscape:
- Bharat Biotech & University of Wisconsin: Bharat Biotech launched India’s first dedicated cell and gene therapy facility at Genome Valley, Hyderabad, expanding its expertise beyond vaccines into regenerative and personalised medicine. The company is collaborating with University of Wisconsin to develop next-generation CAR therapies using AI
- PM-STIAC Initiative: The Prime Minister’s Science, Technology & Innovation Advisory Council (PM-STIAC) is driving government-industry partnerships to accelerate cell and gene therapy commercialisation, focusing on rare diseases and cancer treatments
- NexCAR19 Launch: Hinduja Hospital, Khar, partnered with ImmunoACT to bring NexCAR19 (Actalycabtagene autoleucel)- India’s first homegrown CAR-T cell therapy to cancer patients in Mumbai
- NKure Therapeutics and CRISPR Therapeutics: Co-develop CTX112, an allogeneic (donor-derived) CAR-T therapy targeting CD19-positive B-cell cancers. Unlike traditional CAR-T, CTX112 uses CRISPR gene editing to create an off-the-shelf solution with faster turnaround and lower cost
- Miltenyi Biotec and Translational Health Science and Technology Institute (THSTI): The partnership aims to boost R&D, technology transfer, training, and capacity building to make CGT more accessible and affordable in India
- Caring Cross and ImmunoACT: This partnership aims to develop and commercialise TriCAR-T cell therapy in India for leukemia and lymphoma. Unlike conventional CAR-T therapies that target a single antigen, TriCAR-T targets multiple tumor antigens, reducing the risk of relapse due to antigen escape
- Qartemi CAR-T Launch Milestone: Immuneel Therapeutics launched Qartemi, India’s first globally benchmarked CAR-T therapy for B-cell Non-Hodgkin Lymphoma, developed in collaboration with Hospital Clínic de Barcelona. With strong clinical results and lower pricing, it marks a major step in making advanced cancer therapies more accessible in India
Recent Advancements in India
India is making strides in gene therapy and cell-based treatments:
- CAR-T Therapy for Solid Tumours: Indian researchers presented evidence of CAR-T therapy effectiveness against gastric and brain tumors at the American Society of Clinical Oncology (ASCO) 2025 conference, highlighting India's growing expertise in solid tumor immunotherapy
- Nagpur’s First CAR-T Therapy Centre: A private hospital in Nagpur successfully performed CAR-T therapy for blood cancer patients, marking a major leap in regional cancer care
- Hemophilia-A Gene Therapy Trial: A lentiviral-transduced autologous gene therapy trial in Tamil Nadu has demonstrated restoration of Factor VIII production, offering hope for hemophilia patients
- NexCAR19 Clinical Results: The IIT Bombay–Tata Memorial–ImmunoACT collaboration reported showed a 73 per cent response rate in leukemia and lymphoma patients, demonstrating strong clinical efficacy
- Bharat Biotech CGT Facility Milestone: Bharat Biotech inaugurated a $75 million state-of-the-art Cell and Gene Therapy facility in Hyderabad’s Genome Valley, focused on producing viral vectors (AAV, lentivirus, adenovirus) essential for cell and gene therapies
- Cellogen Bi-Specific CAR-T Milestone: Cellogen Therapeutics, a Delhi-based biotech startup, secured a patent for India’s first indigenously developed bi-specific 3rd generation CAR-T cell therapy. Unlike conventional CAR-T therapies that target a single antigen, Cellogen’s platform targets two tumor-specific antigens simultaneously
Cost and Reimbursement Landscape
In India, CAR-T therapy (such as NexCAR19) is available at approximately Rs 50 lakh (about $60,000) including hospital fees. This pricing is more than 80 per cent lower than the $500,000–$700,000 range in countries like the United States, and substantially lower compared to options available in Europe and China.
However, the Government of India is taking gradual steps toward reducing the financial burden. Recent press releases and governmental factsheets indicate that pilot schemes under Ayushman Bharat are being considered to eventually cover high-cost procedures - including therapies like CAR‑T for conditions such as relapsed leukemia.
Gene therapy costs in India vary, with pricing models evolving to include outcomes-based reimbursement and staged payments. The Indian government is exploring financial assistance programmes to improve affordability.
Conclusion
India’s cell and gene therapy sector is rapidly evolving, driven by academic research, biotech innovation, and government support. With affordable CAR-T therapy, expanding gene therapy trials, and cutting-edge advancements, India is poised to become a global hub for immuno-cell and gene therapy.
Sudeep Krishna, Co-Founder & President, Healthark Insights










