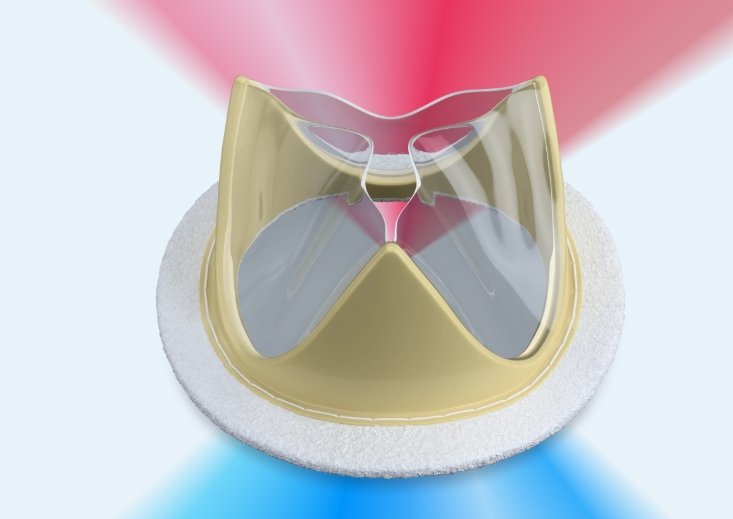
Foldax® announces launch of TRIA™ mitral valve in India
September 03, 2025 | Wednesday | News
First time a polymer heart valve has been made commercially available
US-based Foldax® Inc., a leader in the development of innovative polymer heart valves, in conjunction with its local manufacturing partner Dolphin Life Science LLP, has announced the launch of the TRIA™ Mitral Valve in India, marking the first time a polymer heart valve has been made commercially available to patients anywhere in the world.
Mitral valve disease is a concern in India, with rising surgical volumes and increased need for long-term treatment solutions. The TRIA™ Mitral Valve is positioned to offer a new option for patients seeking a durable solution without the lifestyle limitations of mechanical valves.
As part of the launch, Foldax has initiated the Evolve™ Training & Certification Programme to ensure high-quality outcomes and surgeon preparedness. Physicians who complete training will be certified to implant the valve at participating hospitals.
The TRIA Mitral Valve in the United States is limited to investigational use only, while it is approved for use in India by CDSCO.
“We are honoured to work with India’s top cardiovascular surgeons and Dolphin Life Science to bring next generation medical solutions to the Indian population,” said Laxmikant Khanolkar, Senior Vice President of Foldax Asia and MEA. “This is the first in a series of heart valve solutions we intend to introduce into the market.”