Nandani Medical Laboratories inks partnership to advance intelligent pharma quality in India
July 24, 2025 | Thursday | News
To strengthen quality assurance with advanced inspection technology
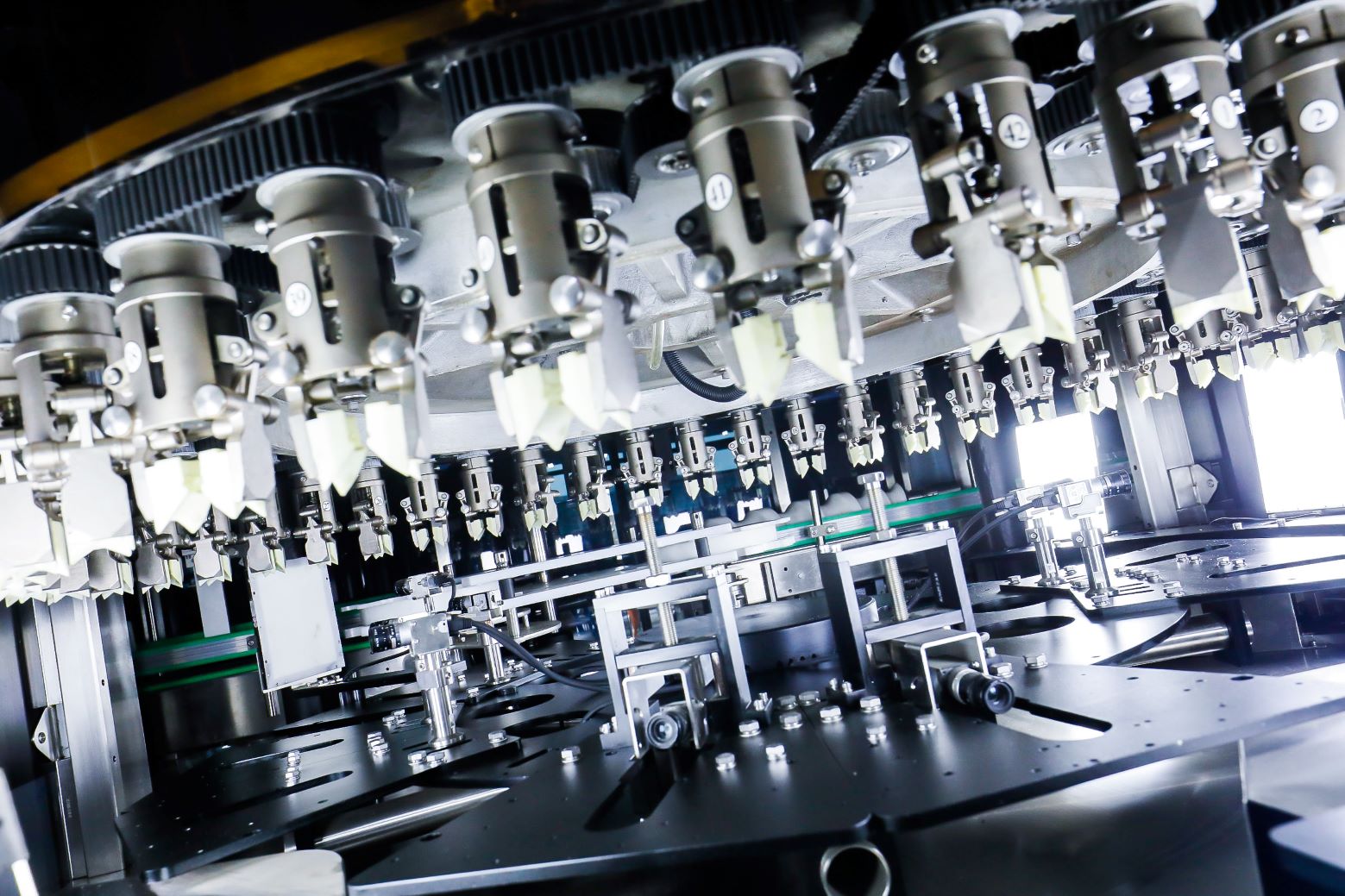
China's Xin Weisheng and Indore-based Nandani Medical Laboratories are collaborating to advance intelligent pharma quality in India. Xin Weisheng is contributing to India’s growing pharmaceutical quality standards through its AI-enabled visual inspection systems.
These systems bring accuracy, speed, and consistency to manufacturers seeking to meet evolving global regulatory benchmarks.
With clients across 40+ countries, Xin Weisheng’s global footprint is further solidified through its enduring partnership with India-based Nandani Medical Laboratories—a WHO-GMP, ISO 9001:2015 certified manufacturer of ampoules, liquid vials, and dry powder injections specialising in human and veterinary injectable formulations.
The AI-enabled machines operate at high speeds of 400–600 bottles per minute, and are capable of detecting a broad spectrum of defects without the need for manual pre-programming. The result: a 70% improvement in detection efficiency versus traditional visual inspection, allowing Nandani to boost throughput while minimising quality risks.
Dr Anil Kharia, Director at Nandani Medical Laboratories, said, “Our collaboration with Xin Weisheng has allowed us to integrate cutting-edge automation into our production lines without compromising agility or reliability. This has been key in ensuring batch consistency and regulatory confidence as we expand our global reach.”