WHO prequalifies Mylan HIV self-test
July 09, 2019 | Tuesday | News
Mylan and Atomo Diagnostics announce WHO prequalification approval
image credit- istock.com
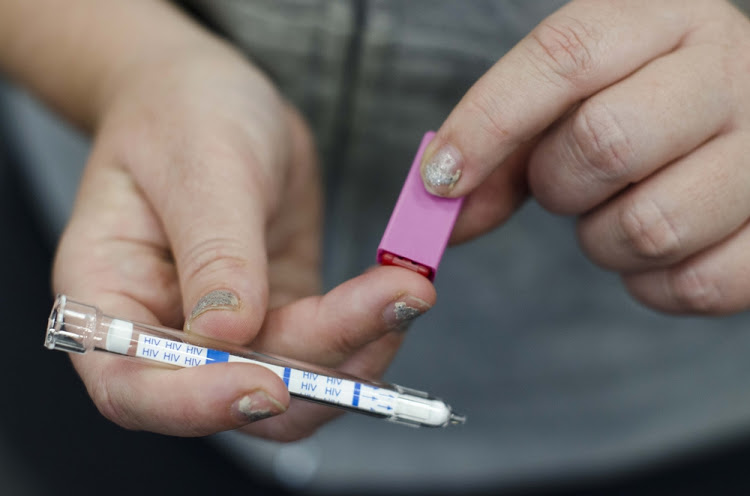
Global pharmaceutical company Mylan N.V. and medical device manufacturer Atomo Diagnostics have announced that the Mylan HIV Self Test, a handheld in vitro HIV rapid diagnostic test for self-testing, has received Prequalification approval by the World Health Organization (WHO PQ).
The WHO PQ process includes a rigorous evaluation of the test's technical performance and manufacturing sites. Prequalification signifies that the product meets global standards of quality, safety and performance and is a signal to global funders and Ministries of Health that the product can be procured.
The Mylan HIV Self Test detects the presence or absence of HIV antibodies through a fingerstick. It uses one-fifth the volume of blood necessary for other tests and provides a result in just 15 minutes.
The convenience and discretion afforded by self-testing enables individuals to test in the comfort and privacy of their own home, making the test an effective way of reaching hard-to-reach populations. In September 2018, Mylan announced a partnership with Atomo Diagnostics, the manufacturer of the test, covering more than 100 countries across Africa, Asia, the Middle East, the Commonwealth of Independent States (CIS), and Latin America.