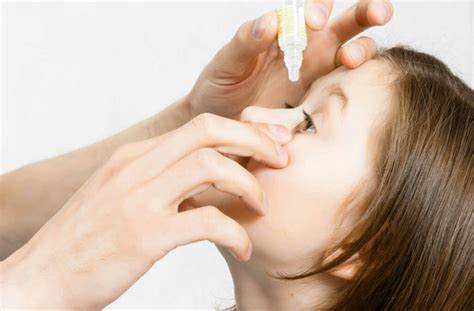
Entod Pharma secures CDSCO approval for eye drop to slow down myopia progression in children
April 25, 2025 | Friday | News
Following the successful completion of Phase 3 clinical trials in India
image credit- shutterstock
Mumbai-based Entod Pharmaceuticals has received marketing authorisation from the Central Drugs Standard Control Organisation (CDSCO) for a new eye formulation developed to slow the progression of myopia in children aged 6 to 12 years. This marks the first global regulatory approval for this unique treatment strength of an eye medicine specifically for paediatric use.
This milestone follows the successful completion of Phase 3 clinical trials in India and a rigorous approval process by India’s apex drug regulator. The treatment will be available strictly by prescription from a registered medical practitioner for children diagnosed with myopia, following an evaluation by an eye doctor.
Dr Mohita Sharma, Medical Director at Tirupati Eye Centre & Research Institute and a principal investigator in the Indian clinical study, commented, "Myopia, or near sightedness, is a growing public health concern, especially among school-aged children with increased exposure to prolonged near work and digital screens. This new treatment is a significant advancement in paediatric eye care, offering a means to effectively slow the progression of myopia after detailed examination to determine the child's suitability for this treatment, and to help prevent complications associated with high myopia and improve long-term visual outcomes."
Commenting on the CDSCO’s marketing approval, Nikkhil K Masurkar, CEO of Entod Pharmaceuticals, stated, "With myopia rates in India rising from 4% in 1999 to nearly 25% today, and projections suggesting that by 2050 one in two children could be affected, the need for such a therapy has never been more urgent."